The laboratory requires tumor material from the patient for testing. A transport kit is provided for shipping. The transport kit should be present before the tumor is removed.
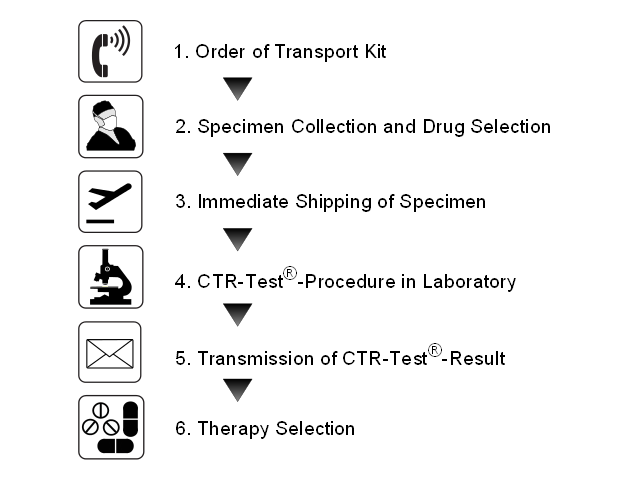
As a service to clinics in Germany, we offer an extensive support during the first use of the CTR-Test®:
An employee of TherapySelect is going to guide the transport kit to the site of specimen collection. In case of a surgery he could attend this surgery in order to solve emerging issues immediately. Afterwards, he will directly transport the tumor and documents, which are completed in advance, to the laboratory.
1. Order of Transport Kit
At the very latest 1-2 days before specimen collection the CTR-Test® should be ordered from TherapySelect:
Phone: + 49 - 6221 - 8936 - 152
An employee notes the most important data and sends out a transport kit for the CTR-Test®. This can be either a transport kit for solid tumor or a transport kit for malignant effusion. After ordering by phone the transport kit for the CTR-Test® is going to be sent out, so that it will be available for the date of specimen collection.
Two kinds of transport kits for the CTR-Test®:
Transport kit for solid tumor

- stable outer packaging (cardboard)
- stable inner packaging (polystyrene)
- transport tube containing transport medium
- cool pack(s)
- forms for testing (not in the picture)
- transport bag (not in the picture)
- (air) waybill (not in the picture)
Transport kit for malignant effusion

- stable outer packaging (cardboard)
- stable inner packaging (polystyrene)
- leakage protection bag including materials for adding of heparine
- cool pack(s)
- forms for testing (not in the picture)
- (air) waybill
 2. Specimen Collection and Drug Selection
2. Specimen Collection and Drug Selection
Please note before getting started:

- Patient must not have had chemotherapy or radiation therapy within 2 weeks of specimen collection.
- Store the transport tube in refrigerator (+4°C) until usage.
- Freeze cool pack(s) in freezer (-20°C) at least 24 h before surgery.
- Organize specimen pick-up one day before surgery. Contact the logistic company according to additional provided country specific information.
For questions call TherapySelect (Tel: +49-6221-8936-152). - Please fax completed forms Laboratory Service Requisition
 and Patient Consent and Patient Order (credit card)
and Patient Consent and Patient Order (credit card) or Patient Consent and Patient Order (SEPA)
or Patient Consent and Patient Order (SEPA) to TherapySelect (Fax: +49-6221-8936-153). On the Laboratory Service Requisition, the treating physician fills out the most important patient details and selects therapeutic agents to be tested. Further details can be found on the site Drug Selection. Patient Consent and Patient Order have to be filled out by the patient.
to TherapySelect (Fax: +49-6221-8936-153). On the Laboratory Service Requisition, the treating physician fills out the most important patient details and selects therapeutic agents to be tested. Further details can be found on the site Drug Selection. Patient Consent and Patient Order have to be filled out by the patient.
 Specimen collection of solid tumor:
Specimen collection of solid tumor:
The surgical removal of the tumor occurs during a stay in the hospital. Because the CTR-Test® depends on a sufficient number of living malignant cells its functioning is limited by the following aspects.
- Surgically Removed, Fresh Tumor Material
In order to get high quality results, it is important that only fresh, living samples are shipped immediately after specimen collection. Avoid any microbiological contamination. Since living cells are required, there must not be formalin-fixation or freezing of the specimen. During specimen collection or at the pathology, the tumor sample is sterilely put into the provided specimen transport tube, which is closed tightly. Afterwards, the inner and outer specimen tubes have to be labeled with relevant patient details.
- Sufficient Amount of the Specimen
About 1 g of tumor tissue is needed to carry out the CTR-Test® (smaller amounts are often enough, too). A reference for the size of the sample might be a cube with approximately 1 cm edge length.
Specimen might also originate from metastases of lymph nodes.
See also: Specimen Handling Instructions![]()
Specimen collection of malignant effusion:

- Analysis of ascites (accumulation of fluid inside the abdomen) or pleural effusion (accumulation of fluid between the pleura and the lung membrane) is possible as well. These fluids usually contain enough tumor cells, which can be extracted from the fluid for testing.
- An amount of 250 ml is needed. Please bear in mind that the risk, that the sample does not contain enough living tumor cells, is higher for malignant fluids than for solid tumors. Specimen collection is usually carried out by puncture, whereat 250-2000 ml of fluid are collected in a sterile evacuation bottle or polyethylene bag and provided heparine is added before it is closed. As for solid tumors, specimens have to be shipped immediately after collection.
See also: Specimen Handling Instructions![]()
3. Immediate Shipping of Specimen
The logistic company contacted in advance will pick up the tumor sample after specimen collection at the agreed time and location. Transport of tumor specimen should ideally happen as fast as possible. Therefore, shipping on the same day is important. The tumor is usually delivered to the laboratory on the next morning. Below, you can find the steps necessary for packing and shipping of specimen for both kinds of transport kits.
Packing and shipping of solid tumor:

- Put the transport tube into the transport box.
- Place the cool pack(s) on top.
- Close the transport box (including the polystyrene lid).

- Put the filled out “Laboratory Service Requisition” and “Patient Consent and Patient Order” form into the transport bag.
- Put the transport box in the provided transport bag and seal it.
- Ship specimen on the day of collection.
- Ensure that package and signed (air) waybill is present for pick-up at arranged time and site.
Packing and shipping of malignant effusion:

- Place secretion bag (put in leakage protection bag) in the transport box inside the polystyrene container.
- Add frozen cool pack(s).
- Close polystyrene box with polystyrene lid.
- Put the completed forms (“Laboratory Service Requisition” and “Patient Consent and Patient Order”) into the transport box onto the polystyrene lid.
- Seal the (cardboard) transport box.
- Ship specimen on the day of collection.
- Ensure that package and signed (air) waybill is present for pick-up at arranged time and site.
4. CTR-Test®-Procedure at the Laboratory

The tumor specimen is normally delivered to the laboratory by the logistic company on the next morning. Immediately after arrival the specimen is processed and the CTR-Test® is started. Only if difficulties regarding the specimen or the documents occur the physician or patient will be contacted by TherapySelect on the same day. An explanation of the different steps of the CTR-Test® procedure can be found under CTR-Test®-Procedure.
5. Transmission of CTR-Test®-Result

Usually the CTR-Test® is finished 7-9 days after arrival of the tumor at the laboratory and the test result is transmitted to the treating physician after a final quality control. The test result is sent by mail and can also be sent via email and/or fax if requested. In the email the result will be attached as a password-proteced document. Of course, TherapySelect is available for any open questions. The test result is explained in detail under CTR-Test®-Result.
6. Therapy Selection

For the therapy of the cancer patient there are different therapeutic options available. These options are discussed on the site Cancer Therapies. Now the physician has the difficult task to select the most successful therapy for an individual patient from the variety of therapy options. In particular for the selection of drugs the CTR-Test® can be an important aid. Besides the test result, the physician has to consider further information, which are mentioned under CTR-Test®-Result. One or more substances might be a good option according to the test result, but in the context of the individual disease, other therapies might be more reasonable. The final decision has to be made by both, the treating physician and the patient, hoping that they have selected the best therapy.
In general, diagnostic products are tools the physician can use for therapy selection.