What is Guardant360®?

Guardant360® - An Overview
Guardant360® is a liquid biopsy which is offered by the company Guardant Health (http://www.guardanthealth.com). We decided to collaborate with Guardant Health in order to provide our customers an additional possibility for planning their cancer therapy.
Guardant360® is a laboratory test, which is performed with a blood sample. Patients are suitable for this test if they suffer from a solid tumor in an advanced stage (stage III or IV) and are planned to be treated with targeted drugs.
Liquid biopsy is especially an option for patients
- For whom no biopsy can be performed
- Whose tumor is unobtainable
- For whom no sufficient tumor tissue is available for an adequate biological evaluation of the tumor
- For whom archived biopsies or results are outdated
- For whom one or more interventions have occured since the last biopsy
In contrast to the CTR-Test® or tumor profiling the liquid biopsy is non-invasive, which means no intervention (surgery or biopsy) is necessary, only some blood has to be drawn.
Via liquid biopsy it can be determined before start of a therapy, which targeted drugs could lead to a positive response in individual patients. Thereby ineffective therapies are avoided and valuable time is saved during therapy.
Additionally patients are suitable for Guardant360® who want to perform a monitoring and aftercare of their cancer therapy with the help of liquid biopsy.
How does Guardant360® work?
Efficacy test for targeted drugs
In the blood of a cancer patient in an advanced stage there is cellfree DNA from cancer cells, which detached from the tumor and circulates in the blood (ctDNA, circulating tumor DNA). This ctDNA is gained from the blood sample of a patient and analysed via a moleculargenetic method (sequencing) if specific clinical relevant alterations of the genes on the DNA (mutations) are present.
Simply put, cancer occurs when the DNA of a normal cell in the body is damaged and thus mutations arise. These mutations lead to an uncontrolled division of cells and this finally leads to the development of a tumor.
Targeted Therapies were developed to attack tumors at specific mutations. These mutations are point mutations, indels, fusions and amplifications of genes. The presence of specific mutations plays a role in the response to corresponding targeted drugs. After performing the liquid biopsy, by means of the presence of specific mutations in a patient it can be assumed which targeted drugs could be an effective therapy option and which drugs will probably not work. For the effective drugs both already approved targeted drugs and drugs in a clinical trial are regarded. Therefore patients are given the opportunity to inform about suitable clinical trials and perhaps taking part in them.
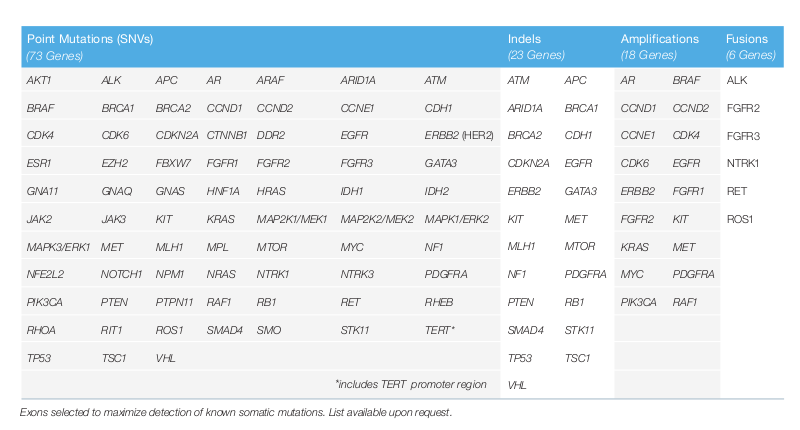
The following 73 genes of the patients are analyzed by Guardant360® for point mutations (all 73 genes), indels (23 genes), amplifications (18 genes) and fusions (6 genes). This list is updated if new research findings are available.
The latest list you can find on the homepage of Guardant Health (http://www.guardanthealth.com/).
Monitoring and aftercare of a cancer therapy
Guardant360® can be applied for the monitoring and aftercare of a cancer therapy as well. Via the liquid biopsy not only present mutations are detected but also the level of present ctDNA in general is determined. Thereby without a big intervention it can be periodically observed if a tumor responds to a corresponding therapy with targeted drugs, chemotherapeutics or any other cancer therapy or not. When a high level of tumor cells is present in the body there is also a higher level of ctDNA in the blood. If a tumor responds to a therapy and decreases, the level of ctDNA in the blood is also less or even there is no ctDNA present anymore.
Furthermore in case of progression or when there is a tumor that is not responding to therapy after some time, it can be examined if probably further mutations came along, which are relevant for therapy planning. It can happen during a cancer therapy that a tumor develops strategies, so-called resistance mechanisms, via further mutations to avoid therapy and continue to spread.
Advantages and quality criteria of Guardant360®
- Guardant360® is the first clinically validated comprehensive liquid biopsy that is available and allows a comprehensive molecular analysis based on the evaluation of ctDNA.
- It is used by oncologists in leading cancer centers and more than 1000 hospitals worldwide. More than 40,000 patients have been sequenced.
- Numerous studies and publications verify the benefit of Guardant360® for therapy planning of cancer patients (https://guardanthealth.com/clinical-studies/). Compared to other liquid biopsies Guardant360® has more peer-reviewed publications, including clinical outcome studies.
- The laboratory of Guardant Health is CLIA-certified and CAP-accredited.
- Since a tumor is heterogenous and within a tumor there could be different types of cancer cells, it could be that when doing a normal biopsy only a specific type of cancer cells is taken out and not all present mutations of the tumor are detected. When these cancer cells afterwards are tested for the efficacy of drugs, a drug is found that is effective against these cancer cells. However it could be that there are other cancer cells in the tumor with additional mutations which do not respond to the therapy and continue to spread. With liquid biopsy all types of present mutations of the cancer cells are detected at the same time and thus the therapy can be adapted much better.
- In case of progression newly formed mutations can be detected.
- Contrary to tests where tumor tissue is needed, with Guardant360® a comprehensive non-invasive genotyping of cancer patients can be performed even when a normal biopsy is not possible or biopsy material is not sufficient.
- With Guardant360® (repeated) painful, expensive and risky biopsies are avoided since only 2 vials (10 ml) of blood are required. Therefore it can be perfomed frequently when required.
- The concentration of ctDNA within the blood is very low. Standard sequencing techniques are not specific and sensitive enough to detect mutations of the ctDNA correctly. The secured digital sequencing method of Guardant360® is 1000x more accurate than standard sequencing methods and results in a 99.9999% specificity. Nearly all false positive results are eliminated.
- Guardant360® identifies all measurable somatic genomic targets, which are recommended by the guidelines. 73 genes are sequenced and all 4 main types of alterations (point mutations, amplifications, fusions and indels) are detected. These genes encode for targetable proteins. Not only common hotspots but also whole exons are sequenced.
- In 85% of the cases mutations are found. These could lead to three different results regarding single targeted drugs in the report: 1) FDA approved indication for this tumor, 2) FDA approved indication for other tumors and 3) trials available.
- The results of the liquid biopsy suggest physicians and patients available treatment options (approved therapies and clinical trials), which are customized to the specific cancer of a single patient. This indicates that Guardant360® is used for the area of personalized medicine.
- Guardant360® can also be applied for the monitoring and aftercare of a cancer therapy.
Guardant360® - Interview with Helmy Eltoukhy, CEO