Find the Right Cancer Diagnostic
TherapySelect develops and distributes cancer diagnostic products in order to extend survival times of cancer patients and to improve their quality of life.
Our diagnostic services can be used individually or together.
For the decision the following questions need to be answered:
- Is it a solid tumor or leukemia / lymphoma?
- What is the current stage of the cancer disease?
- What kind of tumor material from the patient is available?
- Which drug efficacy is of interest and should be evaluated?
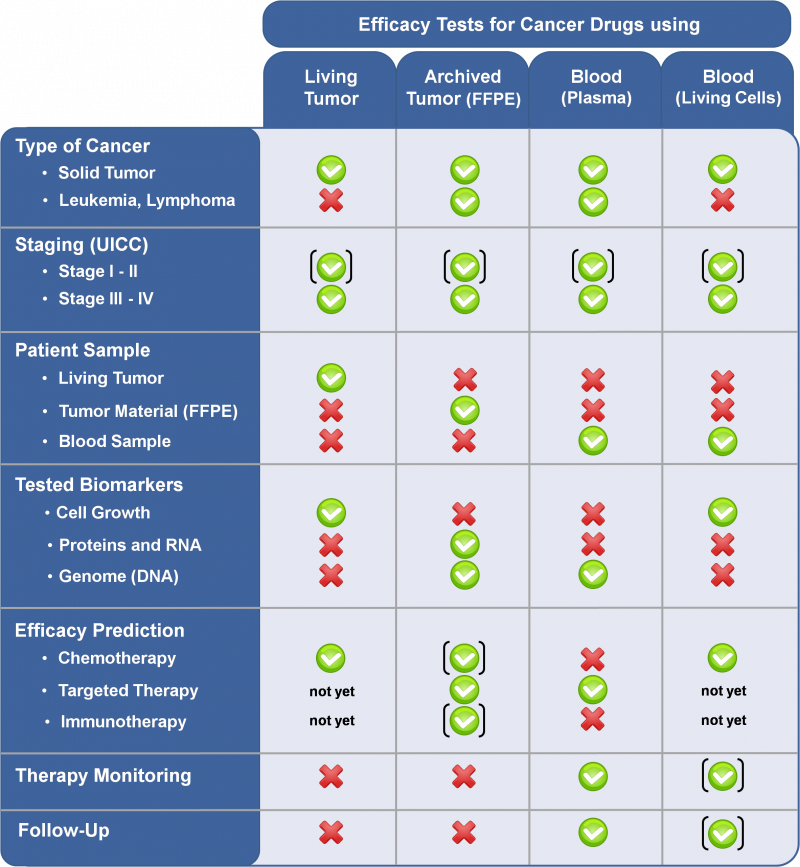
The table below is a selection guide to find the right diagnostic tool for the relevant cancer. The type of patient (tumor) sample is decisive for the prediction of efficacy and the diagnostic test to be used.
Explanations to the table:
- Symbols:
- A green check indicates that the diagnostic can be used.
- A red cross indicates that the diagnostic cannot be used.
- Brackets around the green checks indicate that the test cannot be used without restriction.
- At an early tumor stage the efficacy tests are only useful if a drug therapy is reasonable.
- For the efficacy prediction the testable drugs are limited.
- Patient Sample:
- A solid tumor is a tumor that arises in body tissue. For example breast, colon, ovary, lung or prostate cancer (to name a few).
- Living tumor is either solid tumor tissue or malignant effusion, which is sent to TherapySelect directly after removal from the patient (via surgery, biopsy or puncture) and is being processed in a way that living tumor cells can be used for the test.
- Tumor material FFPE (Formalin Fixed, Paraffin Embedded) is removed tumor tissue that is fixed in formalin and embedded in paraffin. Here no living tumor cells are present. It is also called archived tumor material. This is normally stored in pathology.
- Efficacy Prediction:
- The targeted therapies do also include anti-hormone drugs.
- The efficacy prediction can also include the prediction of resistance.
- For targeted therapies and immunotherapies the CTR-Test® is not yet validated.
Which Drug Efficacy Can Be Determined by Which Test?
The actual number of drugs (in particular the new targeted drugs) increases steadily. Up to now there are over 40 approved chemotherapeutic drugs and over 40 approved new targeted drugs available. In addition there are over 200 targeted drugs which are currently being evaluated in clinical trials. Even to the latter drugs a patient has accesss if he fulfills the inclusion/exclusion criteria of the particular trial and is accepted by a center. That means there are manifold drug alternatives. Our diagnostics can help to select the best therapy strategy.
Please request from us an overview of testable chemotherapeutic and targeted drugs.
Overview of the Diagnostic Services
CTR-Test® (Chemotherapy-Resistance-Test with living tumor)
Identification of ineffective agents before the start of a chemotherapy
A functional test that uses living tumor specimen to analyze the response of a tumor to a certain agent.
PCDx™ (Personalized Cancer Diagnostic with tumor material)
Determination of potentially effective, targeted and selected chemotherapeutic drugs to optimize the therapy
This service correlates molecular biomarker data generated from patient’s tumor, with molecular biomarker/drug associations derived from the cancer clinical literature. PCDx™ provides oncologists with the most up to date, clinically actionable information to help them individualize treatment for solid tumor cancer patients. Formalin-fixed, paraffin-embedded biopsy material is the preferred biological material to be used for testing.
Determination of potentially effective targeted drugs for optimization of the therapy strategy and monitoring and aftercare of the cancer therapy
Guardant360® is a diagnostic for liquid biopsy. This laboratory test is performed with a blood sample of cancer patients in an advanced stage (stage III or IV) in order to find a suitable therapy option for a therapy with targeted drugs. The liquid biopsy is especially useful for patients, where no biopsy can be performed or where no sufficient tumor material is available for a biological evaluation of the tumor.
In addition Guardant360® can be used for monitoring and aftercare of a cancer therapy.
The Essential Differences between the Diagnostic Services
- The CTR-Test® is able to test conventional chemotherapeutic agents – also called cytostatic agents (see also Cancer Therapies). In principle, the test is able to test all agents that directly affect tumor cells, but it is only validated for conventional cytostatic agents. Exceptions are tamoxifen and imatinib (Gleevec®), which are validated as well and can therefore be tested.
- The CTR-Test® needs about 1 gram of living tumor tissue. Tumor tissue or malignant effusion should be transported to the laboratory over night.
- The CTR-Test® takes 7 - 9 days starting from the arrival of specimen at the laboratory.
- For PCDx™ this tumor tissue is analyzed with molecular methods to receive molecular biomarker data generated from patient’s tumor. These data are then correlated with molecular biomarker/drug associations derived from the cancer clinical literature to evaluate the efficacy of new, targeted and selected chemotherapeutic drugs (see also Cancer Therapies). This evidence based tumor profiling service helps the physician to identify and evaluate potential treatment options and develop an individual treatment plan for a specific cancer.
- PCDx™ can be performed using small amounts of fixed (not living) tumor tissue. Ideal tissue for testing is a formalin-fixed, paraffin-embedded specimen.It is preferable to use recent or fresh biopsy material. If this is not possible alternatively archived material, which is stored at the local pathology, may be used for testing. The decision which tissue should be used needs to be discussed with the attending physician.
- About 2 weeks after arrival of the tumor sample in the laboratory the result is complete.
- Using Guardant360® the efficacy of targeted drugs can be tested. The efficacy of chemotherapeutics cannot be tested.
-
Guardant360® only requires a blood sample (liquid biopsy).
For the performance of the test 2 vials with 10 ml of blood are required.
Thus Guardant360® can be used for patients where it is difficult or impossible due to several reasons to obtain sufficient biopsy material for an examination.
- About 2 weeks after arrival of the blood sample in the laboratory the result is complete.
Additionally:
Guardant360® can be applied for monitoring and aftercare of a cancer therapy. It can be investigated via the present level of ctDNA if a therapy is efficient. Newly formed mutations as a reaction of the tumor to the therapies can be detected.